This work studied deactivation mechanism through the oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) on alpha- and beta-MnO2 using density functional theory (DFT) and computational hydrogen electrode (CHE) model. The highlighted results are that
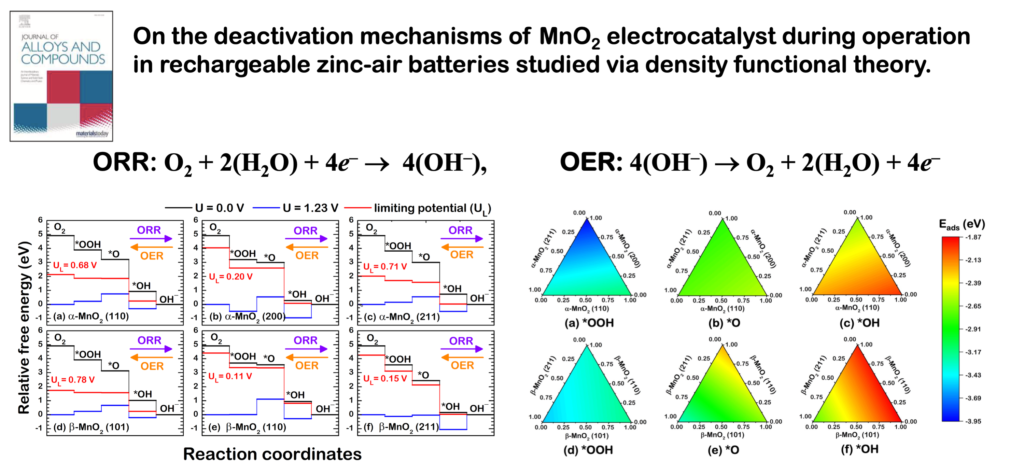
- It is evident that the greater part of the fresh MnO2 surface is α-MnO2 (211), while the spent one is β-MnO2 (110).
- The fresh catalyst consisting mainly of α-MnO2 (211) exhibited high ORR activity, while the spent catalyst β-MnO2 (110) facet dominated displaying lower activity.
- All adsorbate species (*OOH, *O, *OH) act as electron acceptors making the Mn active sites and their neighboring atoms distorted due to the reduced charge of Mn from Mn(IV) to Mn(III).
- ORR on all surfaces except β-MnO2 (211) is terminated by *OOH.
- The poor ORR activity found on β-MnO2 causes the formation of *OOH rather than the final product
Read this research article: https://www.sciencedirect.com/science/article/abs/pii/S0925838821006885
